In genetics, a generation is assumed to represent 20 years. Because the 2003 SARS epidemic and 2019 SARS pandemic are approximately 20 years apart, we can assume they are a generation apart -- for the sake of genetics. There are several misconceptions about the current fight against COVID-19 and ensuing aftermath once a vaccine is found. The original source of the 2003 SARS epidemic caused by the coronavirus known as SARS-CoV was confirmed to be zoonotic, originating from an animal reservoir that includes horseshoe bats (Vespertilio ferrum-equinum), masked palm civets (Paguma larvata), and pangolins (Manis pentadactyla). The current COVID-19 pandemic caused by SARS-CoV-2 is also likely to be zoonotic. Therefore, as long as humans continue to hunt for fresh meat following a hard-day's work clearing out a forest in the Amazon or continue to cage live animals at meat and seafood markets, the risk of pandemics from zoonotic sources will continue to be a major threat to humans. There is every reason to believe that there could possibly be a COVID-40, COVID-60, or COVID-80 every generation. The WHO's acronym COVID represents CoronaVIrus Disease 2019, and if the influezna virus is involved it the next pandemic it could well be INVID-40, INVID-60, or INVID-80.
The current race to find drugs, namely antivirals, to treat patients who are ill and bed-ridden with COVID-19 disease involves identification of molecules that can bind with what is called the active site of the main protease of SARS-CoV-2. While there are other targets of viral proteins, proteases are enzymes that act like scissors to cleave or cut up larger proteins into smaller ones, which are used to manufacture more virions which exit the cell to infect other cells. The main protease of SARS-CoV and SARS-CoV-2 is a chymotrypsin-like protease called 3CLpro, which is important for processing viral proteins and controlling replicase complex activity. As such, 3CLpro is an ideal target for the design of antiviral therapies because of its vital role in viral replication and infection. If drugs can be identified that bind to the active site of 3CLpro and block its catalytic activity, then they will likely minimize viral replication and slow down the infection rate leading to shortened patient recovery times. This approach is required for therapy at the advanced stage of infection near the latter end of the causal pathway. The approach taken by use of a vaccine introduces an antigen or a small segment of a protein of SARS-CoV-2 which the immune system would recognize and memorize as being foreign, and attack upon exposure to similar proteins in the future. The vaccine approach obviates consideration of any part of the causal pathway since disease would not occur after vaccination.
An additional approach for fighting viral pandemics is based on homeopathic consumption of natural products known to have antiviral properties, such as the natural plant-derived flavonoids. Flavonoids are well known for their beneficial effects on neurodegenerative disease, type 2 diabetes, atherosclerosis, cardiovascular disease, and cancer, which have been empirically established in molecular laboratory-based research and human clinical trials. Flavonoids have also shown a wide range of antiviral benefits, since antiviral properties have been established for poliovuris, astrovirus, HIV, enterovirus, respiratory syncytial virus, parainfluenza virus type 3, and influenza virus type A. Laboratory studies with flavonoids have also shown that the proteolytic activity of the original SARS-CoV 3CLpro was inhibited by the flavonoids apigenin, luteolin, quercetin, amentoflavone, quercetin, daidzein, puerarin, epigallocatechin, epigallocatechin gallate, gallocatechin gallate, and kaempferol. However, the lag-time between the 2003 SARS epidemic and appearance of publications on the inhibitory effect of many flavonoids has been as long as 16 years. So, while more research is needed for establishing the inhibitory effects of flavonoids on SARS-CoV-2, there is no guarantee such information will become available over the next decade.
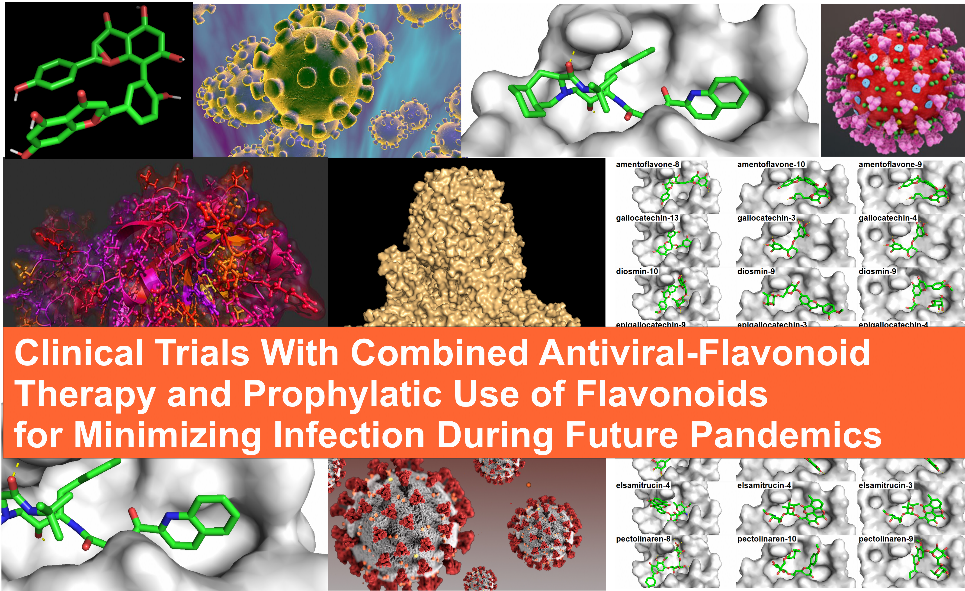
In a repurposing study we performed last month, it was observed that that the flavonoid diosmin ranked 22 among 4,634 drugs in terms of its binding strength at the active site of 3CLpro of SARS-CoV-2. In its energy-minimized state, diosmin was able to deeply penetrate and fully cover the width of the active site's pocket better than 99% of the drugs studied. Better yet, it formed 9 hydrogens bonds, which is a sign that diosmin could have an exceedingly high binding affinity for the active site. In that same study, it was also observed that the green tea flavonoid called epigallocatechin gallate (EGCG) bound to the active site better than 70% of the top 30 antivirals. As a totally relevant aside, we also noted that Remdesavir was one of the worst binding antivirals, and closely related antivirals like Bictegravir and Indinavir were predicted to bind more strongly to the active site of 3CLpro.
Since two flavonoids were predicted to bind significantly to the active site of 3CLpro, we then studied binding patterns for 72 flavonoids, including diosmin and EGCG. Results indicated that the top 10 flavonoids were amentoflavone, gallocatechin gallate, diosmin, epigallocatechin gallate, hidrosmin, catechin gallate, elsamitrucin, pectolinaren, quercetin, and isoquercetin. Other flavonoids investigated with significant binding energies were hesperidin, rutin, rhoifolin, and peurarin. Many of the flavonoids have also been reported in other in silico docking studies of 3CLpro. Altogether, 14 flavonoids have now been identified in multiple in vitro and in silico studies: amentoflavone, daidzein, diosmin, epigallocatechin gallate, gallocatechin gallate, herbacetin, hesperidin, luteolin, naringin, peurarin, pectolinarin, quercetin, rhoifolin, and rutin. Such a finding is monumental in terms of opening new modalities for fighting future viral pandemics, because it means that widely available plant flavonoids found in the common diet may have a role in minimizing viral infection rates, and hence provide a form of prophylactic protection. Many of these flavonoids are available as supplements by themselves or are a component of a natural herb. Diosmin, luteolin, quercetin, and rutin are available as a supplement by themselves. Others are available in raisins, green tea extract, Ginko Biloba, and oil and rinds of the Bergamot orange, found in the black tea known as Earl Grey.
Because flavonoids are ubiquitously available in the common diet as a non-prescription supplement, existing antiviral trials for COVID-19 disease should consider opening one or more additional arms for combination therapy with the antiviral plus a flavonoid. The hypothesis would be that the combined therapy shortens recovery times when compared with the antiviral(s) used alone. The primary outcome would be improved efficacy in the combined therapy arm vs. the single antiviral treatment arm -- or other arms with a variety of doses. New group sequential and adaptive clinical trials using combined antiviral and flavonoid therapy could also be rapidly initiated though compassionate use. If flavonoids are found to show a synergistic effect when used in combination with an antiviral, then they could be consumed in moderation by the public for prophylactic protection from future SARS-CoV-2 infection. Since flavonoids have been consumed in the diet for thousands of years, safety should not be a major issue if the appropriate formularies for dosing and frequency have been established.